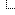Actually Rob, it's the differences in
nuclear binding energy that causes the energy release in fission and fusion. Nuclear binding energy relates to the strong nuclear force that holds neutrons and protons together to make a nucleus. Electrons don't really figure into it.
I attached a chart of binding energy vs # of nucleons (protons + neutrons) in the nucleus. The binding energy is given in MeV per nucleon. MeV is millions of electron-volts, a standard energy unit used in physics. 1 MeV = ~1.6 x 10^-13 Joule. The peak binding energy is Iron-56, and the curve slopes down both ways from there, both for larger atoms and smaller atoms.
For fission, a U-235 atom at the far right with 235 nucleons splits into two smaller atoms, one typically smaller than the other (there are many elements formed, but for example Krypton-92 and Barium-141), plus spitting out 2-3 neutrons. The Kr and Ba are found to the left of Uranium on the chart so have higher binding energy. So if you sum the plus and minus binding energies, one fission releases about 200 MeV of energy, which is about 3.2x10^-11 Joules. You can do the E=mc^2 calculation to see how much mass is lost. Not much.
Same reasoning for hydrogen - helium fusion, but operating on the left hand side of the chart.
More interesting (?) factoids:
- the 200 MeV from one fission is said to be enough to make a grain of sand visibly jump, if you could arrange for that to happen
- to produce 1 watt of thermal energy (heat) takes about 3x10^10 fissions per second
- a Bruce reactor at high power makes about 2800 thermal megawatts. The efficiency of the steam turbine cycle results in only about 800 electrical megawatts out to the grid. The rest is used to warm Lake Huron.
No more physics lectures unless I get paid!!!

73
Dave, VE3WI